- Your cart is empty
- Continue Shopping
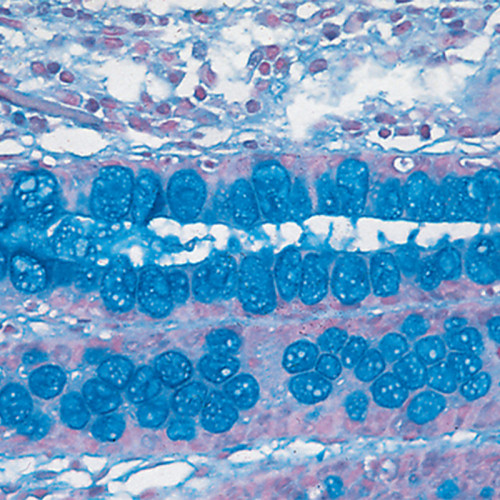
The MGG Quick Stain 100 Test is a rapid staining kit designed for differential staining of blood and bone marrow smears, providing a quick and efficient alternative to traditional May-Grünwald-Giemsa (MGG) staining methods.
🧪 Product Overview
-
Purpose: Rapid differential staining of peripheral blood and bone marrow smears, as well as other air-dried cytological specimens like sediment smears and fine needle aspirates.
-
Method: The kit employs a simplified, manual three-step process that significantly reduces staining time compared to conventional MGG methods.
-
Comparison to Traditional MGG: While traditional MGG staining can take 20–30 minutes, the Quick Stain method offers comparable results in a shorter timeframe, making it suitable for routine laboratory workflows.
🧬 Technical Details
-
Kit Components:
-
Shelf Life: 2 years
-
Storage Conditions: Store at room temperature, away from direct sunlight, in a cool and dry place.
✅ Key Benefits
-
Rapid Processing: Enables specimen processing in as little as 30 seconds.
-
High-Quality Staining: Provides clear differentiation of blood cell types, similar to traditional MGG staining
-
Ease of Use: Simplified manual procedure suitable for routine laboratory use.
-
Versatility: Applicable for various cytological specimens, including blood and bone marrow smears.
🧾 Product Codes
-
ChemBio: CB6110.0100
-
Bio-Optica: 04-090805
-
Diapath: 010253
-
Genprice Distribution: 0707-RRSK27-100






Reviews
There are no reviews yet.